American Hospital Association Disclaimer
The American Hospital Association has not reviewed, and is not responsible for, the completeness or accuracy of any information contained in this material, nor was the AHA or any of its affiliates, involved in the preparation of this material, or the analysis of information provided in the material. The views and/or positions presented in the material do not necessarily represent the views of the AHA. CMS and its products and services are not endorsed by the AHA or any of its affiliates.
Axonics Neuromodulation System For The Treatment Of Urinary Incontinence
Wang et al noted that over-active bladder and UUI affect millions of women and men and results in billions of dollars in health-care expenses. First- and 2nd-line therapy includes behavioral modifications and/or pharmacotherapies however, many patients’ symptoms remain or worsen on these treatments. There has been concern regarding the detrimental side effects of the most widely prescribed medications for these bladder symptom management. As a result, there has been increased interest in continuous sacral neuromodulation, an FDA-approved therapy for refractory UUI. These investigators reviewed current research on the effectiveness and patient/provider satisfaction and safety profile of the Axonics System. Furthermore, they addressed the current state SNM and potential future direction and applicability. The authors concluded that the Axonics system is a safe and effective device for the treatment of OAB and UUI. In additional, it affords patient’s the convenience of a rechargeable, compact, MRI safe system. It should be noted that the rechargeable system, while allowing for approximately 15 years of battery and lead life, may have its challenges in terms of charge burden. Furthermore, this system is easily adapted for experienced implanters of sacral neuromodulating devices.
Treatment Of Urinary Incontinence
Before and immediately following a radical prostatectomy, physicians recommend behavioral modification, a practitioner-guided pelvic floor muscle training program or home exercise program, and pads/diapers/penile clamps/condom catheters to enhance continence recovery. A pelvic floor muscle training program or exercises can also be effective at any point postoperatively.
If urinary incontinence is bothersome after prostate treatment and conservative therapy is not successful, surgery may be considered as early as six months later.
Don’t Miss: Can A Urinary Tract Infection Cause Confusion
Ama Disclaimer Of Warranties And Liabilities
CPT is provided “as is” without warranty of any kind, either expressed or implied, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. No fee schedules, basic unit, relative values or related listings are included in CPT. The AMA does not directly or indirectly practice medicine or dispense medical services. The responsibility for the content of this file/product is with CMS and no endorsement by the AMA is intended or implied. The AMA disclaims responsibility for any consequences or liability attributable to or related to any use, non-use, or interpretation of information contained or not contained in this file/product. This Agreement will terminate upon notice if you violate its terms. The AMA is a third party beneficiary to this Agreement.
How A Prostatectomy Affects Urination
Typically, urine is emptied into the bladder from the kidneys. Two valves called sphincters keep the urine inside the body by remaining closed until the body sends a signal to open them.
When a patient has his prostate completely removed, the surgeon has to remove the prostate gland and one of the sphincters outside the prostate. Having only one sphincter is not usually a problem. Other times, it can affect the nerves and muscles from the surgery and result in urine leakage.
Don’t Miss: Urinary Incontinence In Bed At Night Is
Implantable Sacral Nerve Stimulators
Screening For Urinary Incontinence In Women
Despite the lack of studies determining the benefits and harms of UI screening, the Women’s Preventive Services Initiative recommended that doctors screen women of all ages, including adolescents, for UI yearly by using a questionnaire. The WPSI recommended referring women with UI for further evaluation if it affects their activities and QOL. These recommendations were based on indirect evidence that UI is common, treatment may be effective, and the harms of screening are unlikely to be serious. The recommendations might change if studies directly evaluating the benefits and harms of screening for UI become available. There are no data to support that the correct frequency of screening is yearly .
You May Like: How Long Does Urinary Incontinence Last After Prostate Surgery
How Does It Work
The eCoin Peripheral Neurostimulator System is implanted under the skin of the ankle to stimulate the tibial nerve, a nerve that allows feeling and movement to parts of the leg and foot. This nerve also has some influence over the nerves that control the bladder. Once implanted, the eCoin system delivers electrical pulses to the tibial nerve in 30-minute sessions based on a fixed schedule. A healthcare provider can adjust the level of stimulation based on each patients sensitivity and needs, using the remote control.
Nerves controlling the pelvic organs, including the bladder, originate from the lower spine and are connected to the tibial nerve in the leg, though it is not known exactly how neurostimulation of the tibial nerve helps stimulate the nerves controlling the bladder.
Periurethral Injections Of Bulking Agents
Also Check: Can Sex Cause Urinary Tract Infection
License For Use Of Current Dental Terminology
End User License Agreement:These materials contain Current Dental Terminology , copyright& copy 2021 American Dental Association . All rights reserved. CDT is a trademark of the ADA.
The license granted herein is expressly conditioned upon your acceptance of all terms and conditions contained in this agreement. By clicking below on the button labeled “I accept”, you hereby acknowledge that you have read, understood and agreed to all terms and conditions set forth in this agreement.
If you do not agree with all terms and conditions set forth herein, click below on the button labeled “I do not accept” and exit from this computer screen.
If you are acting on behalf of an organization, you represent that you are authorized to act on behalf of such organization and that your acceptance of the terms of this agreement creates a legally enforceable obligation of the organization. As used herein, “you” and “your” refer to you and any organization on behalf of which you are acting.
Enuresis Not Due To A Substance Or Known Physiological Condition
- 20162017201820192020202120222023Billable/Specific Code
- 20162017201820192020202120222023Billable/Specific Code
Code Also
- 20162017201820192020202120222023Non-Billable/Non-Specific Code
- any associated overactive bladder
- urinary incontinence associated with cognitive impairment
- urinary incontinence NOS
- 20162017201820192020202120222023Billable/Specific Code
Applicable To
- stress incontinence and other specified urinary incontinence
- urinary incontinence NOS
- Applicable To annotations, or
Read Also: Signs Of Urinary Tract Infection In Females
Viveve Announces New Category Iii Cpt Code For Its Stress Urinary Incontinence Procedure
New code approved by American Medical Association and supported by key medical societies
Long-term pathway for potential reimbursement now established for Company’s dual-energy noninvasive treatment for urethral hypermobility to improve stress urinary incontinence in women
ENGLEWOOD, CO / ACCESSWIRE / July 7, 2021 / Viveve Medical, Inc. , a medical technology company focused on women’s intimate health, today announced that the American Medical Association has issued a new Category III Current Procedural Terminology code for the Company’s dual-energy procedure effective January 1, 2022. The new code establishes a pathway for potential reimbursement for Viveve’s noninvasive treatment under evaluation in the PURSUIT trial to improve stress urinary incontinence in women if approved by the U.S. Food and Drug Administration for this indication.
The new Category III CPT code for Viveve’s dual-energy procedure is defined as: endovaginal cryogen-cooled, monopolar radiofrequency remodeling of the tissue surrounding the female bladder neck and proximal urethra for urinary incontinence. Category III CPT codes are AMA approved for new and developing technology, procedures and services. They allow for specific data collection and assessment over time for potential Category I qualification.
About Viveve
For more information visit www.viveve.com.
Safe Harbor Statement
Viveve is a registered trademark of Viveve, Inc.
CPT is a registered trademark of the American Medical Association.
Artificial Urinary Sphincter Device
This three-part implanted device consists of an inflatable cuff/ring around the urethra, a saline-filled balloon next to the bladder, and a scrotal pump. The procedure is typically performed under general anesthesia, and a catheter is inserted to make sure the bladder remains empty during surgery. A perineal incision is made to place the cuff. An inguinal incision is then made to place the balloon and the scrotal pump. When the scrotal pump is manually compressed, the cuff opens and automatically closes after two to three minutes. The device works much like the patients own sphincter.
The single cuff perineal approach is the preferred method. There is also a dual/tandem cuff placement using a transverse scrotal incision, but it increases the risk of complications.
The AUS system is the most reliable and predictable treatment for SUI after prostate treatment. It can successfully treat all degrees of urine leakage and lasts for about seven years.
CPT Codes for AUS Device
Insertion, removal and/or replacement, and repair of an artificial urinary sphincter system are reported with CPT codes 53444-53449.
CPT code 53445 is reported most often.
When a previously inserted AUS system fails, a tandem cuff may need to be inserted to replace the original cuff. When this occurs, 53444 should be assigned.
AUS Coding Example
53447, Removal and replacement of inflatable urethral/bladder neck sphincter including pump, reservoir, and cuff at the same operative session
Also Check: What Do I Take For A Urinary Tract Infection
Moxibustion For The Treatment Of Post
Li and colleagues noted that UI is a frequently identified complication among stroke survivors. Moxibustion is commonly used to treat post-stroke UI in Asian countries. In a systematic review and meta-analysis, these researchers examined the evidence of using moxibustion for post-stroke UI management. A total of 12 databases were searched to identify RCTs using moxibustion to improve post-stroke UI management 4 Chinese journals were also manually screened for potentially eligible articles. A total of 10 studies with 719 subjects and 1 completed trial without published results were included. Compared with “routine methods of treatment and/or care”, the meta-analyses revealed that moxibustion had superior effects in improving UI symptoms and alleviating the severity of UI. The authors concluded that this systematic review identified preliminary research evidence that moxibustion may be effective in managing the symptoms of post-stroke UI these investigators stated that more rigorously designed, large-scale RCTs are needed to provide more robust evidence in this area.
Extraurethral Retropubic Adjustable Compression Devices
Read Also: Parkinson’s And Urinary Tract Infections
The Leva Pelvic Floor Trainer
The Leva pelvic floor trainer is intended for the purpose of rehabilitation and training of weak pelvic floor muscles for the treatment of stress, mixed and mild moderate urge incontinence in women. This device interacts with the user via smart phone technology. There is lack of evidence that the use of this device provides better outcomes than conventional Kegel exercises.
Sacral Nerve Stimulation For Urinary Incontinence
230.18
Please Note: This may not be an exhaustive list of all applicable Medicare benefit categories for this item or service.
Effective January 1, 2002, sacral nerve stimulation is covered for the treatment of urinary urge incontinence, urgency-frequency syndrome, and urinary retention. Sacral nerve stimulation involves both a temporary test stimulation to determine if an implantable stimulator would be effective and a permanent implantation in appropriate candidates. Both the test and the permanent implantation are covered.
The following limitations for coverage apply to all three indications:
- Patient must be refractory to conventional therapy and be an appropriate surgical candidate such that implantation with anesthesia can occur.
- Patients with stress incontinence, urinary obstruction, and specific neurologic diseases which are associated with secondary manifestations of the above three indications are excluded.
- Patient must have had a successful test stimulation in order to support subsequent implantation. Before a patient is eligible for permanent implantation, he/she must demonstrate a 50% or greater improvement through test stimulation. Improvement is measured through voiding diaries.
- Patient must be able to demonstrate adequate ability to record voiding diary data such that clinical results of the implant procedure can be properly evaluated.
10/2001 – Added section as result of a national coverage decision. Effective and implementation dates 01/01/2002.
Also Check: Bacterial Urinary Tract Infection Contagious
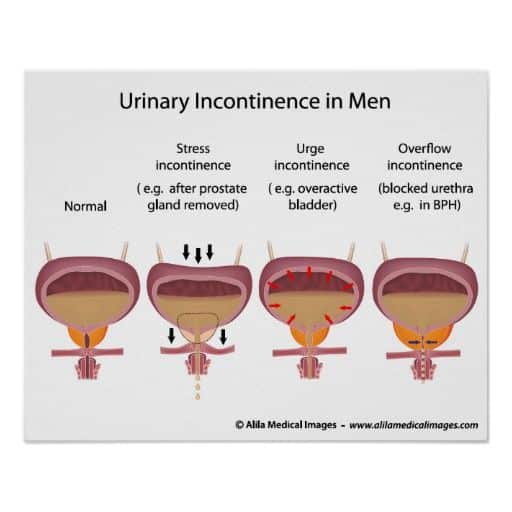
Types Of Urinary Incontinence After Prostate Treatment
The four main types of urinary incontinence after prostate treatment include stress, urge, mixed, and overflow:
- After prostate surgery, stress urinary incontinence is the most common type and involves urine leakage when coughing, laughing, sneezing, or exercising. It is not related to mental stress. The cause is usually a problem with the valve that keeps urine in the bladder sphincter.
- Urge urinary incontinence is a sudden need to urinate.
- Mixed urinary incontinence is a combination of SUI and UUI.
- Overflow urinary incontinence occurs when there is a problem emptying the bladder.
Test Stimulation Of The Interstim
The InterStim product labeling states that, in clinical studies, subjects underwent anywhere from 1 to 6 test stimulation procedures before implantation of InterStim.
The Medtronic InterStim test stimulation lead kit manual stated that Of the 260 patients who qualified for implantation, 169 had a successful result during their first test stimulation procedure. Of the remaining 91 patients, 56 obtained a successful result during a second test stimulation, and 35 obtained a successful result during three or more test stimulations. Reasons for repeat test stimulation procedures included inadequate responses to test stimulation or technical problems . The safety and effectiveness of this therapy has not been established for pediatric use , patients with neurological disease origins, such as multiple sclerosis or diabetes, and bilateral stimulation.
You May Like: Causes Of Urinary Frequency And Urgency In Females
Symptoms For Urinary Incontinence
If you experience one or more of the following symptoms, you should definitely see a doctor:
· Urine leakage when performing normal activities such as exercising, bending, coughing or lifting
· Urine leakage without any warning or urge
· Bed wetting
· Strong, sudden urges to urinate
· Frequent trips to the toilet
Magnetically Controlled Endourethral Artificial Urinary Sphincter
Mazzocchi and colleagues stated that UI is a widespread dysfunction that affects more than 300 million people worldwide. At present, no technological solutions are able to restore continence in a minimally invasive and effective way. These researchers described the design, fabrication, and testing of a novel artificial endourethral urinary sphincter that attempts to fully restore continence. The device can be inserted/retracted in a minimally invasive fashion without hospital admission, does not alter the body scheme and can be applied to both women and men. The device core is a uni-directional polymeric valve and a magnetically activated system, which is able to modulate its opening pressure. Bench tests and ex-vivo tests on a human cadaver demonstrated that the device was able to fully restore continence and allowed urination when desired. The authors concluded that the proposed system showed a high potential as a technological solution that may restore a normal daily life in patients affected by UI. These preliminary findings need to be validated by well-designed studies.
Also Check: Exercises To Help With Urinary Incontinence